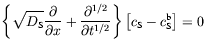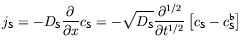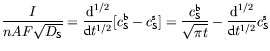Computational & Technology Resources
an online resource for computational,
engineering & technology publications
engineering & technology publications
Civil-Comp Proceedings
ISSN 1759-3433
ISSN 1759-3433
CCP: 84
PROCEEDINGS OF THE FIFTH INTERNATIONAL CONFERENCE ON ENGINEERING COMPUTATIONAL TECHNOLOGY
PROCEEDINGS OF THE FIFTH INTERNATIONAL CONFERENCE ON ENGINEERING COMPUTATIONAL TECHNOLOGY
Edited by: B.H.V. Topping, G. Montero and R. Montenegro
Paper 8
Fractional Differential Equations in Electrochemistry
K.B. Oldham
Department of Chemistry, Trent University, Peterborough, Ontario, Canada
Full Bibliographic Reference for this paper
K.B. Oldham, "Fractional Differential Equations in Electrochemistry", in B.H.V. Topping, G. Montero, R. Montenegro, (Editors), "Proceedings of the Fifth International Conference on Engineering Computational Technology", Civil-Comp Press, Stirlingshire, UK, Paper 8, 2006. doi:10.4203/ccp.84.8
Keywords: electrochemistry, diffusion, semiintegration, semidifferentiation, fractional-operators, electrodes, concentration, flux.
Summary
Fick's laws govern the diffusion of chemical species in aqueous solution and
other media. In one dimension, Fick's second law for the diffusion of a species S
takes the form of the partial differential equation
Here cS, a function of both distance x and time t, is the concentration of species S. DS is a characteristic constant, the diffusivity of S. Consider conditions in which, prior to the time t=0, species S is uniformly distributed throughout the medium, with concentration cSb, then Fick's second law can be written as
In this format, the operator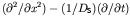 acts on the concentration
difference [cS-cSb], generating zero. In the spirit of the fractional calculus, we can split
the operator into two subsidiary operators, acting sequentially:
acts on the concentration
difference [cS-cSb], generating zero. In the spirit of the fractional calculus, we can split
the operator into two subsidiary operators, acting sequentially:
It is now pertinent to enquire whether there is any circumstance in which the first of these subsidiary operators, acting alone, can convert the concentration difference [cS-cSb] into zero:
It turns out that all that is needed to validate this simplification is for the diffusion field to be semiinfinite. In any such case, Fick's second law, which is second-order in distance and first-order in time, can be replaced by relationship (4), which is first-order in distance and half-order in time. Moreover, one can combine Fick's first law with equation (4) into
where jS is the flux density of species S.
occurs at its an electrode. Species S, the substrate, reaches the electrode by diffusion and species P, the product, leaves by the same diffusive mechanism. The electrons that are liberated constitute the flow of electricity that is manifested as an electric current I. Equation (6) shows that the flux densities of all three participants in the reaction must be mutually proportional at the electrode surface. In fact:
In expression (7), A is the electrode area and F is Faraday's constant (96485 C mol-1). Representing the concentration of species S at the electrode surface by cSs, combination of this result with equation (5) leads to
with a similar result for the product species P. In words, equation (8) reveals that the current flowing through the electrochemical cell is related in a simple fashion to the semiderivative of the concentration of species S at the electrode. An even more useful result comes from semiintegrating equation (8) and its P-analogue. One finds that
showing how measuring the electrochemical current I and semiintegrating it permits one to access to the concentrations of the species at the electrode surface. This ability has proved to be a great asset to electrochemists.
| (1) |
Here cS, a function of both distance x and time t, is the concentration of species S. DS is a characteristic constant, the diffusivity of S. Consider conditions in which, prior to the time t=0, species S is uniformly distributed throughout the medium, with concentration cSb, then Fick's second law can be written as
| (2) |
In this format, the operator
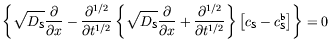 |
(3) |
It is now pertinent to enquire whether there is any circumstance in which the first of these subsidiary operators, acting alone, can convert the concentration difference [cS-cSb] into zero:
It turns out that all that is needed to validate this simplification is for the diffusion field to be semiinfinite. In any such case, Fick's second law, which is second-order in distance and first-order in time, can be replaced by relationship (4), which is first-order in distance and half-order in time. Moreover, one can combine Fick's first law with equation (4) into
where jS is the flux density of species S.
In a typical electrochemical experiment, a reaction such as
occurs at its an electrode. Species S, the substrate, reaches the electrode by diffusion and species P, the product, leaves by the same diffusive mechanism. The electrons that are liberated constitute the flow of electricity that is manifested as an electric current I. Equation (6) shows that the flux densities of all three participants in the reaction must be mutually proportional at the electrode surface. In fact:
In expression (7), A is the electrode area and F is Faraday's constant (96485 C mol-1). Representing the concentration of species S at the electrode surface by cSs, combination of this result with equation (5) leads to
with a similar result for the product species P. In words, equation (8) reveals that the current flowing through the electrochemical cell is related in a simple fashion to the semiderivative of the concentration of species S at the electrode. An even more useful result comes from semiintegrating equation (8) and its P-analogue. One finds that
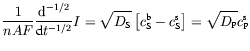 |
(9) |
showing how measuring the electrochemical current I and semiintegrating it permits one to access to the concentrations of the species at the electrode surface. This ability has proved to be a great asset to electrochemists.
purchase the full-text of this paper (price £20)
go to the previous paper
go to the next paper
return to the table of contents
return to the book description
purchase this book (price £105 +P&P)